
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Alantolactone Attenuates Renal Fibrosis via Inhibition of Transforming Growth Factor β/Smad3 Signaling Pathway
- Kyeong-Min Lee, Yeo Jin Hwang, Gwon-Soo Jung
- Diabetes Metab J. 2024;48(1):72-82. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2022.0231
- 1,279 View
- 142 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
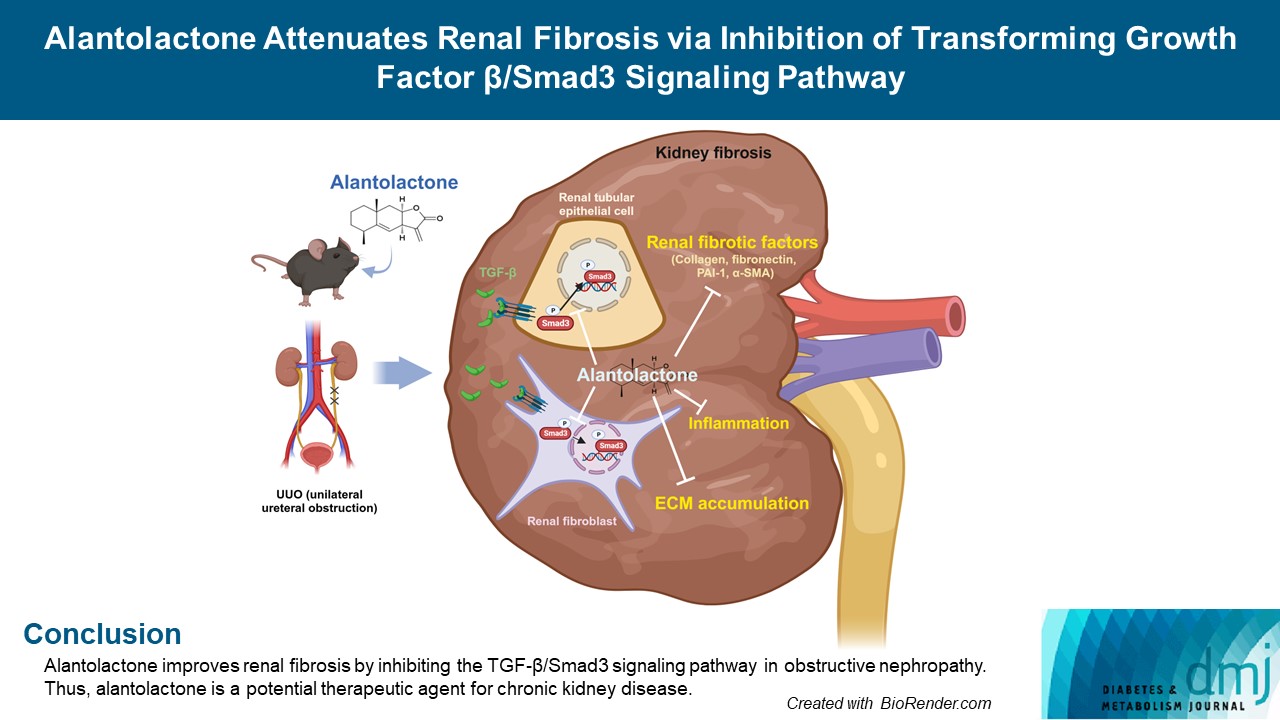
Renal fibrosis is characterized by the accumulation of extracellular matrix proteins and interstitial fibrosis. Alantolactone is known to exert anticancer, anti-inflammatory, antimicrobial and antifungal effects; however, its effects on renal fibrosis remains unknown. Here, we investigated whether alantolactone attenuates renal fibrosis in mice unilateral ureteral obstruction (UUO) and evaluated the effect of alantolactone on transforming growth factor (TGF) signaling pathway in renal cells.
Methods
To evaluate the therapeutic effect of alantolactone, cell counting kit-8 (CCK-8) assay, histological staining, Western blot analysis, and real-time quantitative polymerase chain reaction were performed in UUO kidneys in vivo and in TGF-β-treated renal cells in vitro.
Results
Alantolactone (0.25 to 4 µM) did not affect the viability of renal cells. Mice orally administered 5 mg/kg of alantolactone daily for 15 days did not show mortality or liver toxicity. Alantolactone decreased UUO-induced blood urea nitrogen and serum creatinine levels. In addition, it significantly alleviated renal tubulointerstitial damage and fibrosis and decreased collagen type I, fibronectin, and α-smooth muscle actin (α-SMA) expression in UUO kidneys. In NRK-49F cells, alantolactone inhibited TGF-βstimulated expression of fibronectin, collagen type I, plasminogen activator inhibitor-1 (PAI-1), and α-SMA. In HK-2 cells, alantolactone inhibited TGF-β-stimulated expression of collagen type I and PAI-1. Alantolactone inhibited UUO-induced phosphorylation of Smad3 in UUO kidneys. In addition, it not only decreased TGF-β secretion but also Smad3 phosphorylation and translocation to nucleus in both kidney cell lines.
Conclusion
Alantolactone improves renal fibrosis by inhibiting the TGF-β/Smad3 signaling pathway in obstructive nephropathy. Thus, alantolactone is a potential therapeutic agent for chronic kidney disease.
- Drug/Regimen
- Evogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
- Mi-Jin Kim, Na-young Kim, Yun-A Jung, Seunghyeong Lee, Gwon-Soo Jung, Jung-Guk Kim, In-Kyu Lee, Sungwoo Lee, Yeon-Kyung Choi, Keun-Gyu Park
- Diabetes Metab J. 2020;44(1):186-192. Published online October 31, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0271
- 5,679 View
- 97 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Renal fibrosis is considered to be the final common outcome of chronic kidney disease. Dipeptidyl peptidase-4 (DPP-4) inhibitors have demonstrated protective effects against diabetic kidney disease. However, the anti-fibrotic effect of evogliptin, a DPP-4 inhibitor, has not been studied. Here, we report the beneficial effects of evogliptin on unilateral ureteral obstruction (UUO)-induced renal fibrosis in mice. Evogliptin attenuated UUO-induced renal atrophy and tubulointerstitial fibrosis. Immunohistochemistry and Western blotting demonstrated that evogliptin treatment inhibits pro-fibrotic gene expressions and extracellular matrix production.
In vitro findings showed that the beneficial effects of evogliptin on renal fibrosis are mediated by inhibition of the transforming growth factor-β/Smad3 signaling pathway. The present study demonstrates that evogliptin is protective against UUO-induced renal fibrosis, suggesting that its clinical applications could extend to the treatment of kidney disease of non-diabetic origin.-
Citations
Citations to this article as recorded by- Targeting cluster of differentiation 26 / dipeptidyl peptidase 4 (CD26/DPP4) in organ fibrosis
Birte Ohm, Isabelle Moneke, Wolfgang Jungraithmayr
British Journal of Pharmacology.2023; 180(22): 2846. CrossRef - Linagliptin ameliorates pulmonary fibrosis in systemic sclerosis mouse model via inhibition of endothelial-to-mesenchymal transition
Biwei Pei, Na Zhang, Tingting Pang, Gengyun Sun
Molecular and Cellular Biochemistry.2022; 477(4): 995. CrossRef - Association Between DPP4 Inhibitor Use and the Incidence of Cirrhosis, ESRD, and Some Cancers in Patients With Diabetes
Yewon Na, Soo Wan Kim, Ie Byung Park, Soo Jung Choi, Seungyoon Nam, Jaehun Jung, Dae Ho Lee
The Journal of Clinical Endocrinology & Metabolism.2022; 107(11): 3022. CrossRef - Evogliptin Directly Inhibits Inflammatory and Fibrotic Signaling in Isolated Liver Cells
Hye-Young Seo, So-Hee Lee, Eugene Han, Jae Seok Hwang, Sol Han, Mi Kyung Kim, Byoung Kuk Jang
International Journal of Molecular Sciences.2022; 23(19): 11636. CrossRef - Optimization and validation of a fluorogenic dipeptidyl peptidase 4 enzymatic assay in human plasma
Hyunyee Yoon, Su Hee Cho, Yu Rim Seo, Kyung-Sang Yu, Sung Sup Park, Moon Jung Song
Analytical Biochemistry.2021; 612: 113952. CrossRef - Use of Anti-Diabetic Agents in Non-Diabetic Kidney Disease: From Bench to Bedside
Sungjin Chung, Gheun-Ho Kim
Life.2021; 11(5): 389. CrossRef - Targeting Dermal Fibroblast Subtypes in Antifibrotic Therapy: Surface Marker as a Cellular Identity or a Functional Entity?
Xin Huang, Yimin Khoong, Chengyao Han, Dai Su, Hao Ma, Shuchen Gu, Qingfeng Li, Tao Zan
Frontiers in Physiology.2021;[Epub] CrossRef - Efficacy and safety of evogliptin treatment in patients with type 2 diabetes: A multicentre, active‐controlled, randomized, double‐blind study with open‐label extension (the EVERGREEN study)
Gyuri Kim, Soo Lim, Hyuk‐Sang Kwon, Ie B. Park, Kyu J. Ahn, Cheol‐Young Park, Su K. Kwon, Hye S. Kim, Seok W. Park, Sin G. Kim, Min K. Moon, Eun S. Kim, Choon H. Chung, Kang S. Park, Mikyung Kim, Dong J. Chung, Chang B. Lee, Tae H. Kim, Moon‐Kyu Lee
Diabetes, Obesity and Metabolism.2020; 22(9): 1527. CrossRef Effect of Switching from Linagliptin to Teneligliptin Dipeptidyl Peptidase-4 Inhibitors in Older Patients with Type 2 Diabetes Mellitus
Eugene Han, Minyoung Lee, Yong-ho Lee, Hye Soon Kim, Byung-wan Lee, Bong-Soo Cha, Eun Seok Kang
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 4113. CrossRef- Efficacy and safety of novel dipeptidyl-peptidase-4 inhibitor evogliptin in the management of type 2 diabetes mellitus: A meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Aishwarya Krishnamurthy, LokeshKumar Sharma, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2020; 24(5): 434. CrossRef
- Targeting cluster of differentiation 26 / dipeptidyl peptidase 4 (CD26/DPP4) in organ fibrosis
- Complications
- Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
- Jung Beom Seo, Yeon-Kyung Choi, Hye-In Woo, Yun-A Jung, Sungwoo Lee, Seunghyeong Lee, Mihyang Park, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
- Diabetes Metab J. 2019;43(6):830-839. Published online March 5, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0181
- 5,439 View
- 128 Download
- 23 Web of Science
- 24 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The hypoglycemic drugs dipeptidyl peptidase-4 (DPP-4) inhibitors have proven protective effects on diabetic kidney disease, including renal fibrosis. Although NOD-like receptor protein 3 (NLRP3) inflammasome activation is known to play an important role in the progression of renal fibrosis, the impact of DPP-4 inhibition on NLRP3-mediated inflammation while ameliorating renal fibrosis has not been fully elucidated. Here, we report that the renoprotective effect of gemigliptin is associated with a reduction in NLRP3-mediated inflammation in a murine model of renal fibrosis.
Methods We examined the effects of gemigliptin on renal tubulointerstitial fibrosis induced in mice by unilateral ureteral obstruction (UUO). Using immunohistochemical and Western blot analysis, we quantitated components of the NLRP3 inflammasome in kidneys with and without gemigliptin treatment, and
in vitro in human kidney tubular epithelial human renal proximal tubule cells (HK-2) cells, we further analyzed the effect of gemigliptin on transforming growth factor-β (TGF-β)-stimulated production of profibrotic proteins.Results Immunohistological examination revealed that gemigliptin ameliorated UUO-induced tubular atrophy and renal fibrosis. Gemigliptin-treated kidneys showed a reduction in levels of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, and interleukin-1β, which had all been markedly increased by UUO. In line with the
in vivo Conclusion The present study shows that activation of the NLRP3 inflammasome contributes to UUO-induced renal fibrosis and the renoprotective effect of gemigliptin is associated with attenuation of NLRP3 inflammasome activation.
-
Citations
Citations to this article as recorded by- Novel pharmacological interventions for diabetic kidney disease
Seng Kiong Tan, Jairo A. Pinzon-Cortes, Mark E. Cooper
Current Opinion in Nephrology & Hypertension.2024; 33(1): 13. CrossRef - Integrated analysis reveals crosstalk between pyroptosis and immune regulation in renal fibrosis
Fengxia Bai, Longchao Han, Jifeng Yang, Yuxiu Liu, Xiangmeng Li, Yaqin Wang, Ruijian Jiang, Zhaomu Zeng, Yan Gao, Haisong Zhang
Frontiers in Immunology.2024;[Epub] CrossRef - Di (2-ethylhexyl) phthalate and polystyrene microplastics co-exposure caused oxidative stress to activate NF-κB/NLRP3 pathway aggravated pyroptosis and inflammation in mouse kidney
Shanshan Li, Xuedie Gu, Muyue Zhang, Qihang Jiang, Tong Xu
Science of The Total Environment.2024; 926: 171817. CrossRef - Fluorofenidone attenuates renal fibrosis by inhibiting lysosomal cathepsin‑mediated NLRP3 inflammasome activation
Linfeng Zheng, Wenjuan Mei, Jing Zhou, Xin Wei, Zhijuan Huang, Xiaozhen Lin, Li Zhang, Wei Liu, Qian Wu, Jinhong Li, Yan Yan
Experimental and Therapeutic Medicine.2024;[Epub] CrossRef - HIF1α-BNIP3-mediated mitophagy protects against renal fibrosis by decreasing ROS and inhibiting activation of the NLRP3 inflammasome
Jialin Li, Qisheng Lin, Xinghua Shao, Shu Li, Xuying Zhu, Jingkui Wu, Shan Mou, Leyi Gu, Qin Wang, Minfang Zhang, Kaiqi Zhang, Jiayue Lu, Zhaohui Ni
Cell Death & Disease.2023;[Epub] CrossRef - Pyroptosis in renal inflammation and fibrosis: current knowledge and clinical significance
Ya Liu, Haibo Lei, Wenyou Zhang, Qichang Xing, Renzhu Liu, Shiwei Wu, Zheng Liu, Qingzi Yan, Wencan Li, Xiang Liu, Yixiang Hu
Cell Death & Disease.2023;[Epub] CrossRef - Tubular injury in diabetic kidney disease: molecular mechanisms and potential therapeutic perspectives
Yu Wang, Mingyue Jin, Chak Kwong Cheng, Qiang Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Hederagenin inhibits high glucose‐induced fibrosis in human renal cells by suppression of NLRP3 inflammasome activation through reducing cathepsin B expression
Guohua Yang, Wang Yang, Hairong Jiang, Qing Yi, Wei Ma
Chemical Biology & Drug Design.2023; 102(6): 1409. CrossRef - Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis
Rikke Nørregaard, Henricus A. M. Mutsaers, Jørgen Frøkiær, Tae-Hwan Kwon
Physiological Reviews.2023; 103(4): 2847. CrossRef - Adenine model of chronic renal failure in rats to determine whether MCC950, an NLRP3 inflammasome inhibitor, is a renopreventive
Mahmoud S. Sabra, Fahmy K. Hemida, Essmat A. H. Allam
BMC Nephrology.2023;[Epub] CrossRef - Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome
Yun Zhang, Song Zhang, Bolin Li, Yingchun Luo, Yongtai Gong, Xuexin Jin, Jiawei Zhang, Yun Zhou, Xiaozhen Zhuo, Zixi Wang, Xinbo Zhao, Xuejie Han, Yunlong Gao, Hui Yu, Desen Liang, Shiqi Zhao, Danghui Sun, Dingyu Wang, Wei Xu, Guangjin Qu, Wanlan Bo, Dan
Cardiovascular Research.2022; 118(3): 785. CrossRef - The NLRP3 inflammasome in fibrosis and aging: The known unknowns
Yanqing Liu, Xuezeng Xu, Wangrui Lei, Yuxuan Hou, Yan Zhang, Ran Tang, Zhi Yang, Ye Tian, Yanli Zhu, Changyu Wang, Chao Deng, Shaofei Zhang, Yang Yang
Ageing Research Reviews.2022; 79: 101638. CrossRef - Research progress of endothelial‐mesenchymal transition in diabetic kidney disease
Ying Chen, Hang Zou, Hongwei Lu, Hong Xiang, Shuhua Chen
Journal of Cellular and Molecular Medicine.2022; 26(12): 3313. CrossRef - Exploring the mechanism of Shendi Bushen capsule in anti-renal fibrosis using metabolomics theory and network analysis
Tianwei Meng, Hong Chang, Hongyu Meng
Molecular Omics.2022; 18(9): 873. CrossRef - Gemigliptin suppresses salivary dysfunction in streptozotocin-induced diabetic rats
Wan Seok Kang, Woo Kwon Jung, Su-Bin Park, Hyung Rae Kim, Junghyun Kim
Biomedicine & Pharmacotherapy.2021; 137: 111297. CrossRef - Long‐Term Dipeptidyl Peptidase 4 Inhibition Worsens Hypertension and Renal and Cardiac Abnormalities in Obese Spontaneously Hypertensive Heart Failure Rats
Edwin K. Jackson, Zaichuan Mi, Delbert G. Gillespie, Dongmei Cheng, Stevan P. Tofovic
Journal of the American Heart Association.2021;[Epub] CrossRef - Disulfiram inhibits inflammation and fibrosis in a rat unilateral ureteral obstruction model by inhibiting gasdermin D cleavage and pyroptosis
Yu Zhang, Ruicheng Zhang, Xiaohu Han
Inflammation Research.2021; 70(5): 543. CrossRef - Inflammasome as an Effective Platform for Fibrosis Therapy
Ting-Ting Chen, Feng Xiao, Nan Li, Shan Shan, Meng Qi, Zi-Ying Wang, Sheng-Nan Zhang, Wei Wei, Wu-Yi Sun
Journal of Inflammation Research.2021; Volume 14: 1575. CrossRef - Targeting Dermal Fibroblast Subtypes in Antifibrotic Therapy: Surface Marker as a Cellular Identity or a Functional Entity?
Xin Huang, Yimin Khoong, Chengyao Han, Dai Su, Hao Ma, Shuchen Gu, Qingfeng Li, Tao Zan
Frontiers in Physiology.2021;[Epub] CrossRef - Linagliptin Protects against Endotoxin-Induced Acute Kidney Injury in Rats by Decreasing Inflammatory Cytokines and Reactive Oxygen Species
Tsung-Jui Wu, Yi-Jen Hsieh, Chia-Wen Lu, Chung-Jen Lee, Bang-Gee Hsu
International Journal of Molecular Sciences.2021; 22(20): 11190. CrossRef - Psidium guajava Flavonoids Prevent NLRP3 Inflammasome Activation and Alleviate the Pancreatic Fibrosis in a Chronic Pancreatitis Mouse Model
Guixian Zhang, Liming Tang, Hongbin Liu, Dawei Liu, Manxue Wang, Jun Cai, Weijun Liu, Wei Nie, Yi Zhang, Xiaomeng Yu
The American Journal of Chinese Medicine.2021; 49(08): 2001. CrossRef - Effect and Regulation of the NLRP3 Inflammasome During Renal Fibrosis
Hong Zhang, Zhengchao Wang
Frontiers in Cell and Developmental Biology.2020;[Epub] CrossRef - Zhen-Wu-Tang Protects IgA Nephropathy in Rats by Regulating Exosomes to Inhibit NF-κB/NLRP3 Pathway
Honglian Li, Ruirui Lu, Yu Pang, Jicheng Li, Yiwen Cao, Hongxin Fu, Guoxing Fang, Qiuhe Chen, Bihao Liu, Junbiao Wu, Yuan Zhou, Jiuyao Zhou
Frontiers in Pharmacology.2020;[Epub] CrossRef - Protective effect of exogenous hydrogen sulfide on diaphragm muscle fibrosis in streptozotocin-induced diabetic rats
Rui Yang, Qiang Jia, Yan Li, Shomaila Mehmood
Experimental Biology and Medicine.2020; 245(14): 1280. CrossRef
- Novel pharmacological interventions for diabetic kidney disease
- Complications
- Renoprotective Effect of Gemigliptin, a Dipeptidyl Peptidase-4 Inhibitor, in Streptozotocin-Induced Type 1 Diabetic Mice
- Gwon-Soo Jung, Jae-Han Jeon, Mi Sun Choe, Sung-Woo Kim, In-Kyu Lee, Mi-Kyung Kim, Keun-Gyu Park
- Diabetes Metab J. 2016;40(3):211-221. Published online March 31, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.3.211
- 6,358 View
- 53 Download
- 22 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Dipeptidyl peptidase-4 (DPP-4) inhibitors are widely used in the treatment of patients with type 2 diabetes and have proven protective effects on diabetic kidney disease (DKD). Whether DPP-4 inhibitors have renoprotective effects on insulin-deficient type 1 diabetes has not been comprehensively examined. The aim of this study was to determine whether gemigliptin, a new DPP-4 inhibitor, has renoprotective effects in streptozotocin (STZ)-induced type 1 diabetic mice.
Methods Diabetes was induced by intraperitoneal administration of a single dose of STZ. Mice with diabetes were treated without or with gemigliptin (300 mg/kg) for 8 weeks. Morphological changes of the glomerular basement membrane (GBM) were observed by electron microscopy and periodic-acid Schiff staining. In addition, we measured blood glucose and urinary albumin excretion and evaluated fibrotic markers using immunohistochemical staining, quantitative reverse transcription polymerase chain reaction analysis, and Western blot analysis.
Results Gemigliptin did not reduce the blood glucose levels of STZ-treated mice. In gemigliptin-treated mice with STZ, a significant reduction in urinary albumin excretion and GBM thickness was observed. Immunohistological examination revealed that gemigliptin attenuated renal fibrosis induced by STZ and decreased extracellular matrix protein levels, including those of type I collagen and fibronectin, and Smad3 phosphorylation. In cultured rat renal cells, gemigliptin inhibited transforming growth factor β-stimulated type I collagen and fibronectin mRNA and protein levels via down-regulation of Smad3 phosphorylation.
Conclusion Our data demonstrate that gemigliptin has renoprotective effects on DKD, regardless of its glucose-lowering effect, suggesting that it could be used to prevent DKD, including in patients with type 1 diabetes.
-
Citations
Citations to this article as recorded by- Diabetic fibrosis
Izabela Tuleta, Nikolaos G. Frangogiannis
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2021; 1867(4): 166044. CrossRef - Protective roles of thymoquinone and vildagliptin in manganese-induced nephrotoxicity in adult albino rats
Heba El-Sayed Mostafa, Eman Ahmed Alaa El-Din, Dalia Abdallah El-Shafei, Nehal S. Abouhashem, Aisha Abdallah Abouhashem
Environmental Science and Pollution Research.2021; 28(24): 31174. CrossRef - Evogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
Mi-Jin Kim, Na-young Kim, Yun-A Jung, Seunghyeong Lee, Gwon-Soo Jung, Jung-Guk Kim, In-Kyu Lee, Sungwoo Lee, Yeon-Kyung Choi, Keun-Gyu Park
Diabetes & Metabolism Journal.2020; 44(1): 186. CrossRef - Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
Jung Beom Seo, Yeon-Kyung Choi, Hye-In Woo, Yun-A Jung, Sungwoo Lee, Seunghyeong Lee, Mihyang Park, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
Diabetes & Metabolism Journal.2019; 43(6): 830. CrossRef - Recent advances in the pathogenesis of microvascular complications in diabetes
Sungmi Park, Hyeon-Ji Kang, Jae-Han Jeon, Min-Ji Kim, In-Kyu Lee
Archives of Pharmacal Research.2019; 42(3): 252. CrossRef - Diabetic nephropathy: An update on pathogenesis and drug development
Vikram Rao A/L B Vasanth Rao, Sean Hong Tan, Mayuren Candasamy, Subrat Kumar Bhattamisra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 754. CrossRef - Mechanisms and pathways of anti‐inflammatory activity of DPP‐4 inhibitors in cardiovascular and renal protection
Katarina Tomovic, Jelena Lazarevic, Gordana Kocic, Marina Deljanin‐Ilic, Marko Anderluh, Andrija Smelcerovic
Medicinal Research Reviews.2019; 39(1): 404. CrossRef - Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Jae Hyun Bae, Sunhee Kim, Eun-Gee Park, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Endocrinology and Metabolism.2019; 34(1): 80. CrossRef - The emerging role of novel antihyperglycemic agents in the treatment of heart failure and diabetes: A focus on cardiorenal outcomes
Kelly R. McHugh, Adam D. DeVore, Robert J. Mentz, Daniel Edmonston, Jennifer B. Green, Adrian F. Hernandez
Clinical Cardiology.2018; 41(9): 1259. CrossRef - Acute Kidney Injury and Progression of Diabetic Kidney Disease
Samuel Mon-Wei Yu, Joseph V. Bonventre
Advances in Chronic Kidney Disease.2018; 25(2): 166. CrossRef - Recombinant human GLP-1(rhGLP-1) alleviating renal tubulointestitial injury in diabetic STZ-induced rats
Weiqin Yin, Shiqing Xu, Zai Wang, Honglin Liu, Liang Peng, Qing Fang, Tingting Deng, Wenjian Zhang, Jinning Lou
Biochemical and Biophysical Research Communications.2018; 495(1): 793. CrossRef - Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice
Laura E. Crotty Alexander, Christopher A. Drummond, Mark Hepokoski, Denzil Mathew, Alex Moshensky, Andrew Willeford, Soumita Das, Prabhleen Singh, Zach Yong, Jasmine H. Lee, Kevin Vega, Ashley Du, John Shin, Christian Javier, Jiang Tian, Joan Heller Brown
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2018; 314(6): R834. CrossRef - Renoprotective effect of fucoidan from Acaudina molpadioides in streptozotocin/high fat diet-induced type 2 diabetic mice
Shiwei Hu, Jinhui Wang, Jingfeng Wang, Shijie Li, Wei Jiang, Yu Liu
Journal of Functional Foods.2017; 31: 123. CrossRef - Dipeptidyl peptidase-4 inhibition and renoprotection
Yuta Takagaki, Daisuke Koya, Keizo Kanasaki
Current Opinion in Nephrology and Hypertension.2017; 26(1): 56. CrossRef - Treatment of diabetic kidney disease: current and future targets
Mi-Kyung Kim
The Korean Journal of Internal Medicine.2017; 32(4): 622. CrossRef - Pharmacological Treatment in Diabetes Mellitus Type 1 – Insulin and What Else?
Ewa Otto-Buczkowska, Natalia Jainta
International Journal of Endocrinology and Metabolism.2017;[Epub] CrossRef - GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes
Marcel H. A. Muskiet, Lennart Tonneijck, Mark M. Smits, Michaël J.B. van Baar, Mark H. H. Kramer, Ewout J. Hoorn, Jaap A. Joles, Daniël H. van Raalte
Nature Reviews Nephrology.2017; 13(10): 605. CrossRef - Efficacy, safety and albuminuria‐reducing effect of gemigliptin in Korean type 2 diabetes patients with moderate to severe renal impairment: A 12‐week, double‐blind randomized study (the GUARD Study)
Sun A. Yoon, Byoung G. Han, Sung G. Kim, Sang Y. Han, Young I. Jo, Kyung H. Jeong, Kook H. Oh, Hyeong C. Park, Sun H. Park, Shin W. Kang, Ki R. Na, Sun W. Kang, Nam H. Kim, Young H. Jang, Seong H. Shin, Dae R. Cha
Diabetes, Obesity and Metabolism.2017; 19(4): 590. CrossRef - Sodium butyrate has context-dependent actions on dipeptidyl peptidase-4 and other metabolic parameters
Eun-Sol Lee, Dong-Sung Lee, Prakash Raj Pandeya, Youn-Chul Kim, Dae-Gil Kang, Ho-Sub Lee, Byung-Chul Oh, Dae Ho Lee
The Korean Journal of Physiology & Pharmacology.2017; 21(5): 519. CrossRef - Lobeglitazone, a Novel Peroxisome Proliferator-Activated Receptor γ Agonist, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
Kwi-Hyun Bae, Jung Beom Seo, Yun-A Jung, Hye-Young Seo, Sun Hee Kang, Hui-Jeon Jeon, Jae Man Lee, Sungwoo Lee, Jung-Guk Kim, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
Endocrinology and Metabolism.2017; 32(1): 115. CrossRef - Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus
Sung-Ho Kim, Jung-Hwa Yoo, Woo Je Lee, Cheol-Young Park
Diabetes & Metabolism Journal.2016; 40(5): 339. CrossRef - Risk assessment and management of post-transplant diabetes mellitus
Eugene Han, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Metabolism.2016; 65(10): 1559. CrossRef
- Diabetic fibrosis

 KDA
KDA
 First
First Prev
Prev





